Researching The Causes of Infection-induced Autoimmune Type 1 Diabetes
Identifying a Genetic Phenotype for Low ß-cell Mass and Determining Why Infections Trigger Autoimmunity Against the Pancreas.
Signed in as:
filler@godaddy.com
Identifying a Genetic Phenotype for Low ß-cell Mass and Determining Why Infections Trigger Autoimmunity Against the Pancreas.


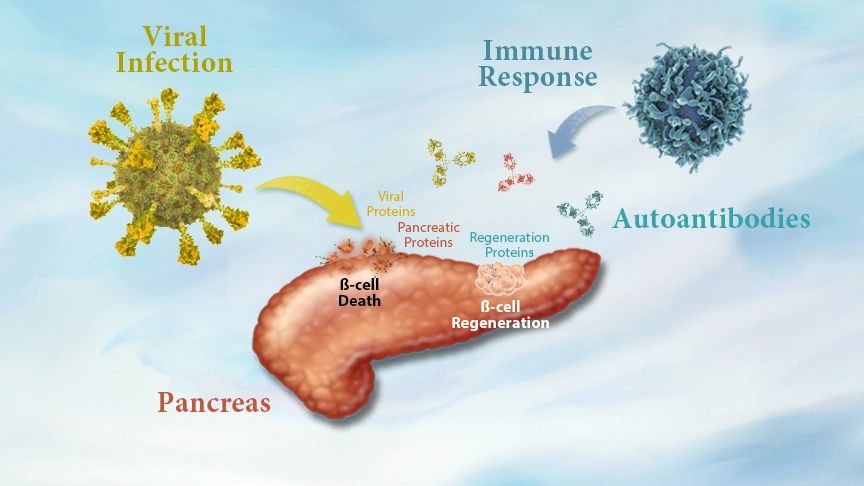
Type 1 Diabetes is believed to be caused by some kind of genetic phenotype and an environmental trigger for chronic autoimmunity, like a viral infection. The combination of both risk factors is associated with declining levels of ß-cell mass caused by increased amounts of ß-cell death. Yet, this loss of ß-cell mass corresponds to increasing rates of ß-cell proliferation.
This apparent contradiction is matched by another. During disease onset, patients will experience declining levels of circulating insulin, yet they will also experience increased insulin resistance. All of this corresponds with the immune system expressing autoantibodies against proteins expressed by ß-cells. One possibility is that pathologic autoantibodies expressed by the immune system may in fact be causing increased rates of ß-cell death.
Type 1 Diabetes: Etiology, Immunology, and Therapeutic Strategies.

Biologic Discovery proposes a modified disease model for Infection-induced Autoimmune Type 1 Diabetes. This disease model defines a genetic phenotype for insufficient stem ß-cell mass caused by low rates of glucose metabolism in the pancreas. According to the proposed disease model, people who express this protein at chronically low levels would define a population of people who are genetically predisposed to ß-cell mass ad suseptible to pancreatic failure following infections that cause ß-cell death.
Loss of ß-cell mass from an infection becomes chronic when the immune system begins to express pathologic autoantibodies against both infectious and pancreatic proteins. One of these autoantibodies is proposed to target a second biomarker of ß-cell function. While its function remains unknown in the pancreas, this second biomarker is known to modulate pathways for cellular differentiation and cell death. Biologic Discovery hypothesizes that loss of this second biomarker during disease onset would accelerate rates of ß-cell death.
Despite the increasing rates of ß-cell death, tissue regeneration is also occurring during disease onset and the weeks after insulin therapy begins.

"An interesting window of opportunity in this respect is the honeymoon phase, a transient remission phase that occurs in up to 60% of T1D patients after initiation of insulin treatment. In this period, insulin doses can be greatly reduced or even withdrawn completely." (X)
While Insulin therapy seems to support the process of ß-cell regeneration, insulin alone is not sufficient to regulate restoration of ß-cell mass because disease progression continues. If other co-factors regulating restoration and protection of ß-cell function can be identified, then it might be possible to someday extend and enhance the "Honeymoon Phase" allowing patients to naturally rebuild ß-cell mass to normal levels.

Biologic Discovery's mission is to experimentally confirm the causes of Autoimmune Type 1 Diabetes and the mechanisms regulating The Honeymoon Phase. Once the mechanism for Autoimmunity in Type 1 Diabetes can be confirmed, Biologic Discovery will then leverage those insights to develop a new diagnostic panel and preventative treatment for Type 1 Diabetes.
Below is a short summary of two potential biomarkers of Infection-induced Autoimmune type 1 Diabetes..

Biologic Discovery proposes two regulatory biomarkers of human ß-cell regeneration. The first increases ß-cell mass by increasing glucose metabolism and insulin expression of precursor stem ß-cell located in the pancreatic ducts.
Biologic Discovery has generated data showing this biomarker is chronically depressed in well-controlled Type
Biologic Discovery proposes two regulatory biomarkers of human ß-cell regeneration. The first increases ß-cell mass by increasing glucose metabolism and insulin expression of precursor stem ß-cell located in the pancreatic ducts.
Biologic Discovery has generated data showing this biomarker is chronically depressed in well-controlled Type 1 Diabetes and positively corresponds with increased ß-cell function in long-term Type 1 Diabetes.

Biologic Discovery further proposes that regulation of human ß-cell regeneration also includes a second naturally occurring biomarker which modulates cellular differentiation pathways associated with cell death.
X-ray crustallography evidence suggests the second biomarker may trigger expression of Autoantibodies targeting multiple protein
Biologic Discovery further proposes that regulation of human ß-cell regeneration also includes a second naturally occurring biomarker which modulates cellular differentiation pathways associated with cell death.
X-ray crustallography evidence suggests the second biomarker may trigger expression of Autoantibodies targeting multiple proteins expressed by ß-cells according to the principles of "molecular mimicry."

Loss of of a protein that prevents ß-cell death from immune expression of its autoantibody during disease onset may help explain increased rates of ß-cell death causing Type 1 Diabetes disease progression.
Biologic Discovery has designed a new experiment to look for evidence of this pathologic autoantibody. Contact us for details.
The proposed disease model for Infection-induced Autoimmune Type 1 Diabetes is very similar to another Infection-induced Autoimmune disease model, PANS/PANDA. This is a neurologic disorder similar to OCD and triggered by bacterial infections. This disease model describes the structural similarity between naturally occurring proteins and bacterial proteins as the trigger for expression of pathologic autoantibodies.
Inhibiting these pathologic autoantibodies has been shown in PANS/PANDAs to improve patient outcomes. Could the same be true for people with developing Autoimmune Type 1 Diabetes?
The proposed disease model for Infection-induced Autoimmune Type 1 Diabetes explains ß-cell regeneration following insulin therapy. Biologic Discovery has data showing this effect in mice and humans.
The proposed proposed mechanism for autoimmunity known as "molecular mimicry" explains why multiple proteins expressed by ß-cells, like GAD65 and Insulin, become targets of the autoantibodies following infections from SARS-CoV2.
The proposed disease model identifies and describes the mechanism for insulin-independent insulin resistance experienced during states of insulin insufficiency which may otherwise contribute to ß-cell dysfunction.
The proposed disease model also explains widespread chronic autoimmunity and long-term disease progression in other organs, like the kidneys.
Patients with Type 2 Diabetes express the biomarker for stem ß-cell mass at high levels. While they are characterized by robust ß-cell mass, the opposite is true in their skin. People with Type 2 Diabetes often experience impaired wound healing. The proposed disease model explains impaired would healing in the dermis following infections that trigger autoimmunity.

A novel disease model for Infection-induced Autoimmune Diabetes now defines multiple unique biomarkers for disease progression. These biomarkers will enable the creation of new ELISA Kit diagnostic panels capable of identifying populations of people at risk of developing Gestational and Type 1 Diabetes. This panel can then identify if a patient has been infected by a virus or bacteria that is causing ß-cell death while also verifying if a pathologic autoantibody targeting ß-cells is causing loss of ß-cell mass.

At the time of diagnosis, hormone replacement of the naturally occurring biomarker for ß-cell death prevention may prove to be a therapeutic strategy to prevent loss of functional ß-cell mass. Following restoration of euglycemia and recovery from an infection, hormone replacement of the biomarker for stem ß-cell mass may prove to be a therapeutic strategy to restore ß-cell mass and insulin expression.
The coordination and use of both naturally occurring biomarkers, or their engineered variants, define a novel treatment strategy for perservation and restoration of ß-cell mass in populations of people genetically predisposed insufficent ß-cell regeneration.

Biologic Discovery envisions a future where children no longer develop Autoimmune Type 1 Diabetes. Detection. Treatments that suppress pathologic autoantibodies that target the pancreas will offer healthcare providers ways to prevent long-term disaease progression in their patients. In the future, endocrinologists will tell anxious parents not to worry after their child's diagnosis with Type 1 Diabetes. They've caught the infections early enough and can save their child's pancreas. An approved treatment using naturally occurring proteins will prevent the loss of functional ß-cell mass and suppress autoimmunity from becoming chronic. Once the immune system clears the infection, hormone replacement therapy can then begin to rebuild their child's pancreas.
Are you passionate about curing Type 1 Diabetes?Can you assist in conducting this experiment? Contact us about tell us about your experience and interests.
Casey Steffen M.Sci. is a biomolecular visualization scientist. He is the owner and operator of Biologic Models, a science communication design studio. Biologic Models works with biotechnology companies around the world to help their research scientists explain the nature of health and disease at the molecular level through molecular visualizations and 3D-printed protein models.
Mr. Steffen has been living with autoimmune Type 1 Diabetes for more than 20 years. When not helping other biotech companies, Mr. Steffen runs Biologic Discovery, a virtual biotech company. Through this entity, Mr. Steffen funds experiments testing his hypothesis for Infection-induced Autoimmune Type 1 Diabetes. It is thanks to his background in virology and his personal interest in Type 1 Diabetes that Mr. Steffen was able to intuit the molecular causes of Infection-induced Autoimmune Type 1 Diabetes.
At the start of the 2019 global Coronavirus pandemic, a client of Biologic Models emailed Mr. Steffen asking for help. Mr. Steffen assisted a team of international research scientists who had been commissioned by the Swedish government to get "get up to speed" on the structural proteins of SARS-CoV2. It was during this experience that Mr. Steffen identified the mechanism used by viruses and bacteria to trigger the immune system to express tissue-specific autoantibodies.

We use cookies to analyze website traffic and optimize your website experience. By accepting our use of cookies, your data will be aggregated with all other user data.